DNA repair mechanisms are emerging as critical players in Huntington's disease, revealing new therapeutic opportunities that could transform how we treat this devastating condition.
In this special UK DRI webinar, internationally recognised researchers Prof Sarah Tabrizi (UK DRI at UCL) and Prof Gabriel Balmus (UK DRI at Cambridge):
- Explore what DNA repair is and why these cellular processes are fundamental to understanding Huntington's disease
- Present the latest breakthroughs from the field showing how DNA repair pathways influence disease onset and progression, including exciting insights from recent clinical studies
- Discuss their own collaborative work pioneering novel interventions to stabilise CAG repeat expansions
A fascinating event bridging fundamental DNA repair biology with cutting-edge therapeutic innovation, offering fresh perspectives on treating Huntington's disease at its molecular roots.
References
Bunting et al (2025). Antisense oligonucleotide–mediated MSH3 suppression reduces somatic CAG repeat expansion in Huntington’s disease iPSC–derived striatal neurons. Sci. Transl. Med.17,eadn4600. DOI:10.1126/scitranslmed.adn4600.
UK DRI news item
Scahill et al (2025). Somatic CAG repeat expansion in blood associates with biomarkers of neurodegeneration in Huntington’s disease decades before clinical motor diagnosis. Nat Med 31, 807–818. https://doi.org/10.1038/s41591-024-03424-6.
UK DRI news item
Goold et al (2021). FAN1 controls mismatch repair complex assembly via MLH1 retention to stabilize CAG repeat expansion in Huntington’s disease. Cell Reports, 36(9) doi: 10.1016/j.celrep.2021.109649.
UK DRI news item

Prof Sarah Tabrizi
Group Leader, UK DRI at UCL
Prof Sarah Tabrizi is an award winning scientist who has published over 420 peer-reviewed publications, has been elected a Fellow of the Royal Society, UK Academy of Medical Sciences and US National Academy of Medicine, co-founded the UCL Huntington’s Disease Centre and helped set up the UK All-Party Parliamentary Group for Huntington's disease (HD).
She leads an internationally recognised basic bench science and translational research team focused on understanding mechanism, validating targets and finding disease-modifying therapies for HD. She was PI on the first successful phase 1/2b trial of an antisense oligonucleotide, and currently serves on several SABs advising industry on the development of potential gene targeting and nucleic acid therapies for Huntington’s disease.
Sarah’s research has been recognised by numerous major prizes including the 2019 Yahr Award, 2022 Osler Medal, 2022 HD Society of America Research Award, the 2022 MRC Millennium Medal, the 2023 Arvid Carlsson Award and in 2024 she was elected both to the Fellowship of the Royal Society and the US National Academy of Medicine.

Prof Gabriel Balmus
Group Leader, UK DRI at Cambridge
Interested in the mechanisms controlling the maintenance of nuclear and mitochondrial genomes in mature neurons, Prof Gabriel Balmus joined the UK DRI at Cambridge in 2018. Obtaining his PhD in Molecular and Integrative Physiology in 2013 at Cornell University, USA, he went on to complete postdoctoral training at the Gurdon Institute at University of Cambridge and the Wellcome Trust Sanger Institute. His work has revealed critical mechanisms by which DNA repair proteins like FAN1 either protect against or promote toxic repeat expansions in Huntington's disease and related disorders, opening new therapeutic avenues. As a UK DRI Group Leader, Gabriel brings his wealth of expertise to research genomic instability in neurodegenerative diseases.
Read more about the Balmus Lab

(Chair) Dr Tom Massey
Group Leader, UK DRI at Cardiff
Dr Tom Massey is a clinical academic Neurologist, whose research focuses on genetic modifiers and therapeutic targets for Huntington’s disease (HD). Dr Massey studied Biochemistry at Cambridge University, before completing his PhD at Oxford in mechanisms of DNA repair. He then trained in Medicine at Oxford University, before beginning a Welsh Clinical Academic Training Fellowship in Neurology in 2013. He was awarded a Clinical Research Training Fellowship from the MRC to develop a programme of research into genetic modifiers of HD. In 2021, he completed his neurology training and became a Consultant Neurologist, and was then award an MRC Clinician Scientist Fellowship to further develop his work on genetic modifiers and therapeutic targets for HD. Dr Massey joined the UK DRI in Cardiff as a Group Leader in 2023.
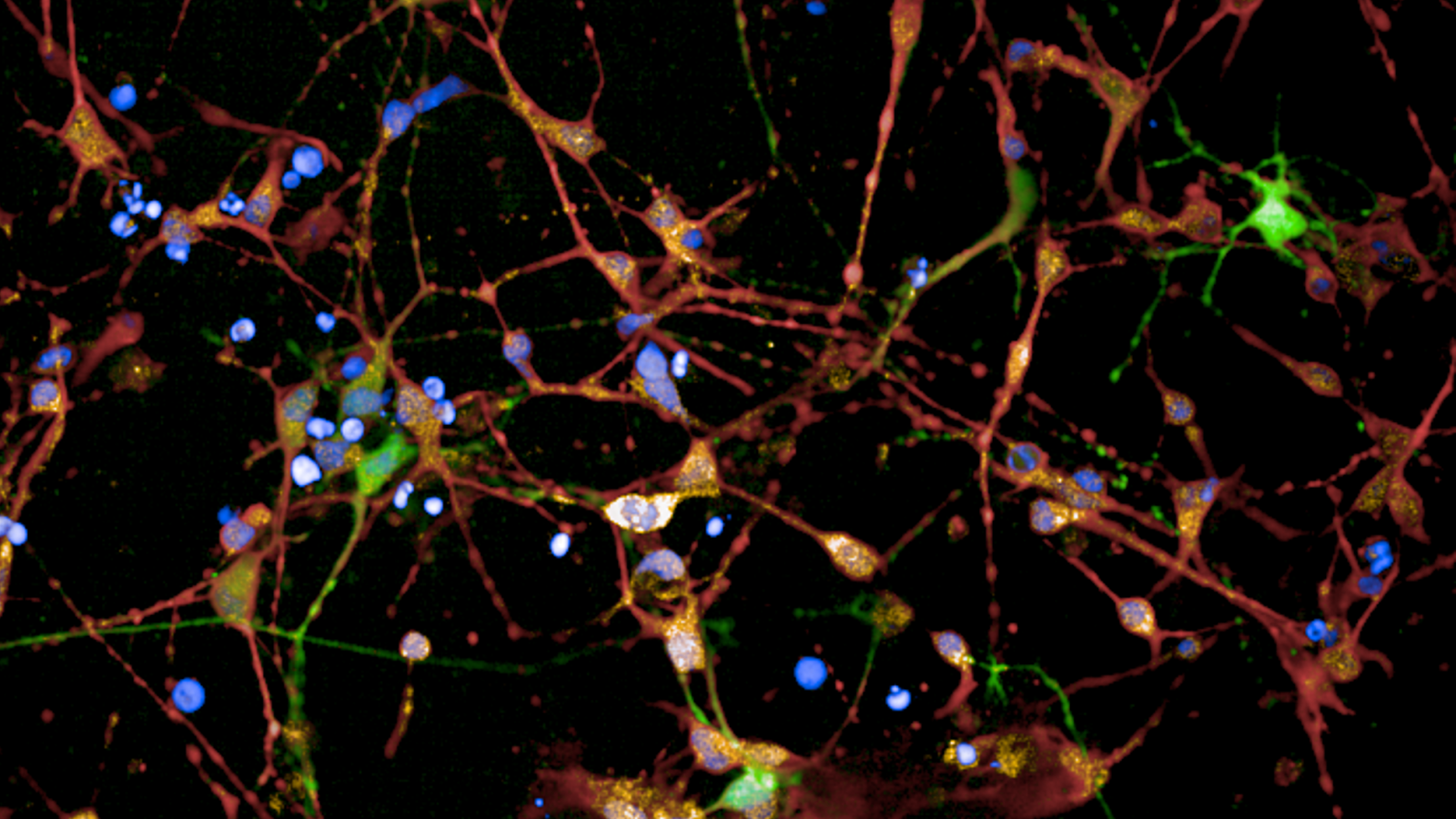
Image courtesy of Prof Sarah Tabrizi - An immunofluorescent staining of HD 125 CAG iPSC–derived striatal neurons after 36 days of differentiation. Costaining of cultured neurons for DARPP32 (green), FOXP1 (orange), MAP2 (red), and Hoechst 33342 counterstain (blue, nuclei) indicates that the cultures were enriched for medium spiny neurons. Marker-positive cell frequency for MAP2, FOXP1, and DARPP32 as a proportion of the total number of cells counterstained with Hoechst was quantified.