Understanding and finding treatments for Alzheimer's and frontotemporal dementia
The Duff Lab focuses on understanding and finding treatments for Alzheimer's disease (AD) and frontotemporal dementia (FTD), which are both linked to harmful changes in a protein called tau. These changes, known as tauopathies, cause damage in the brain and lead to dementia.
The programme has four key goals:
- Understanding genetics: Researchers are studying how genes affect the development of tau-related brain damage. This could help doctors predict how a patient's disease will progress and tailor treatments accordingly.
- Stopping disease spread: The team is investigating how tau-related damage spreads in the brain and looking for ways to stop this process before it leads to severe symptoms like memory loss.
- Protecting vulnerable brain cells: Some brain cells are more prone to damage from tau. The researchers are trying to understand why this happens and how to protect these vulnerable cells.
- Early treatment options: The team is exploring new treatments, including brain stimulation techniques, to intervene in the earliest stages of these diseases, potentially slowing or even reversing their progression.
This work is part of a larger effort to better understand and ultimately cure or prevent dementia.
Latest news

Prof Karen Duff
Prof Karen Duff is a Group Leader and Centre Director at the UK DRI at UCL. Find out more about her career and expertise on her profile page.

Research summary
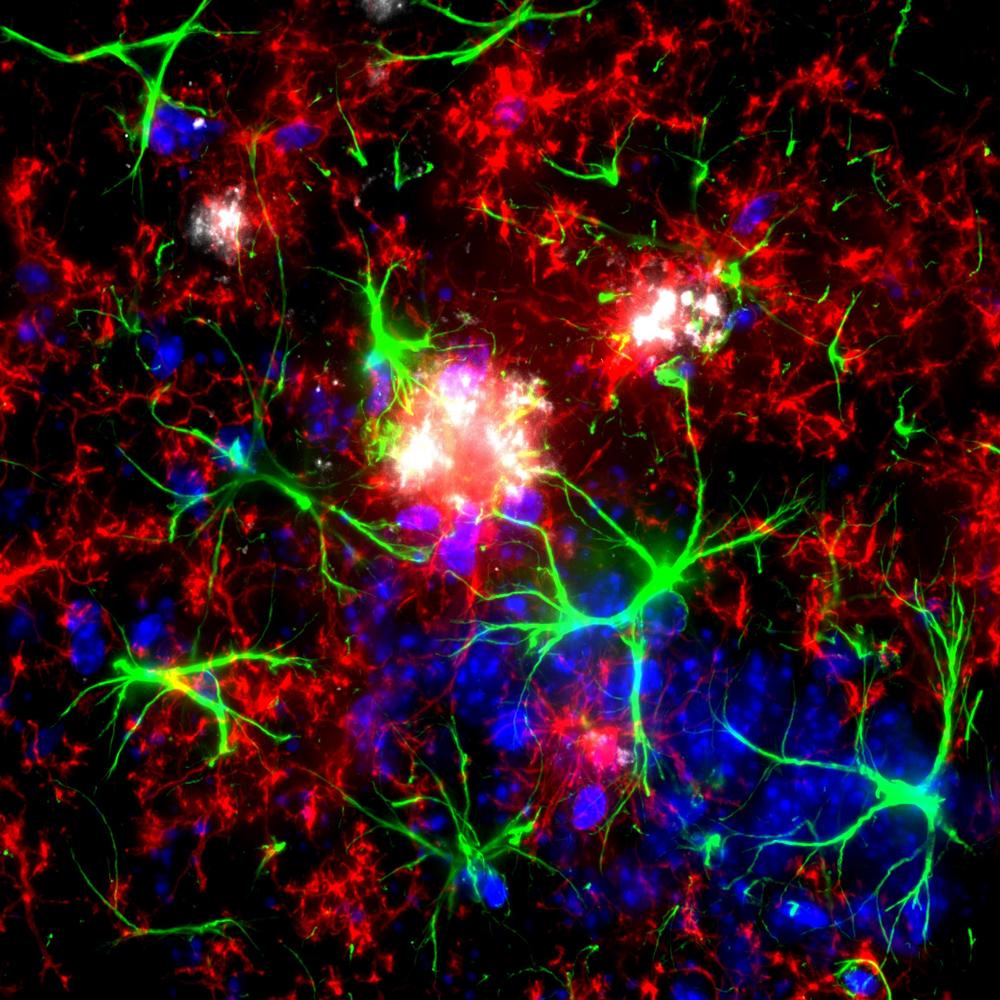
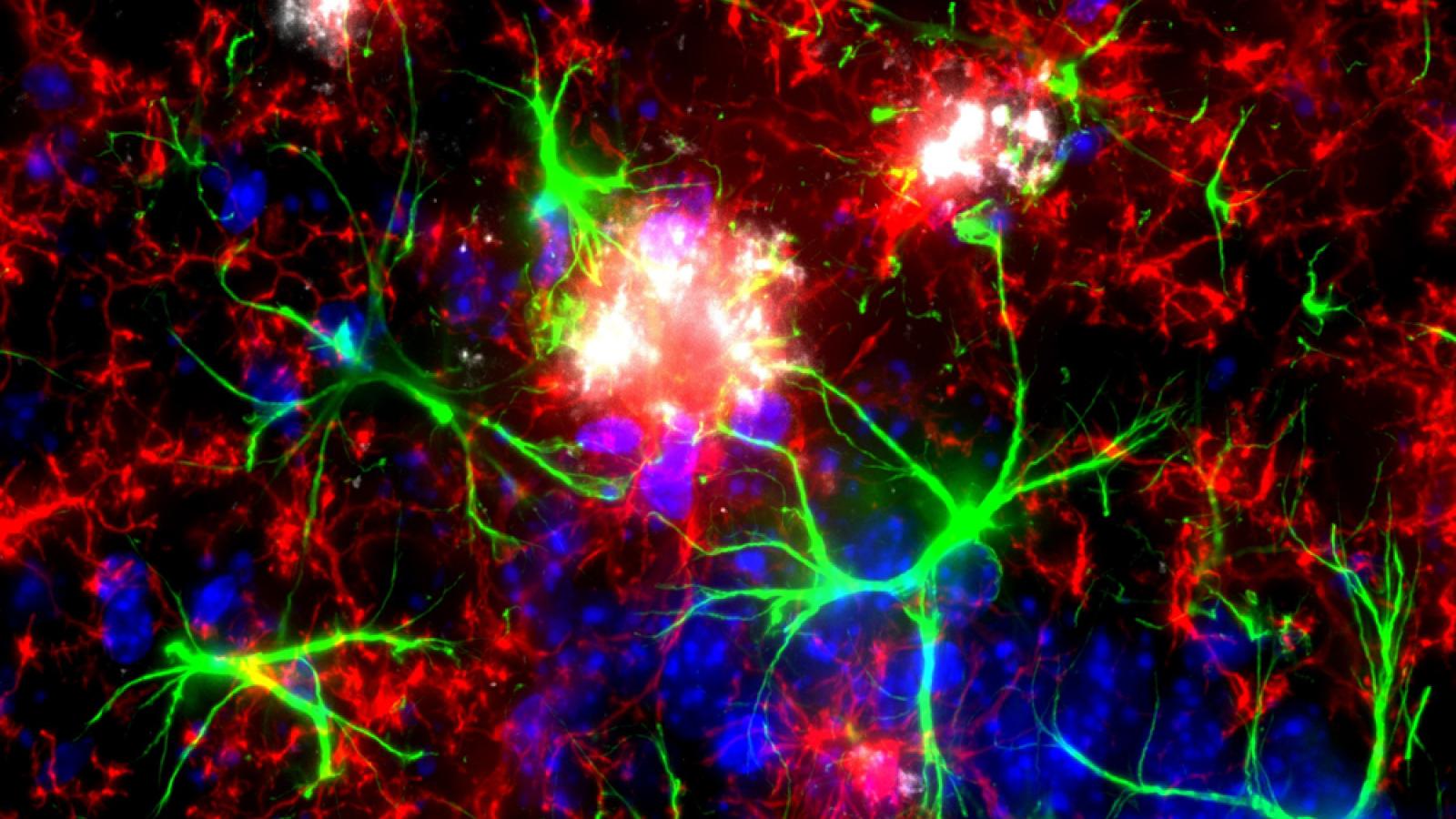
Mouse brain showing microglia (red) and astrocytes (green) are visible surrounding amyloid plaques (white). Credit: Izzie Prankerd, Duff Lab
The molecular causes and consequences of tauopathy in Alzheimer's disease and Frontotemporal dementia
The "pathological tau in AD and FTD" research programme, led by Karen Duff, focuses on uncovering the mechanisms and therapeutic opportunities related to tauopathies - neurodegenerative conditions characterised by the accumulation of tau protein aggregates. Tauopathy is a significant contributor to dementia, including Alzheimer's disease (AD) and Frontotemporal dementia (FTD).
The programme is organised around four main objectives:
- Identifying genetic modifiers: This objective aims to identify and test genetic factors that influence the development and progression of tauopathies. Understanding these genetic influences can improve the prediction of clinical outcomes and allow for more personalized treatments. The research examines how genetic variants affect tau pathology distribution in diseases like progressive supranuclear palsy (PSP) and the cognitive decline associated with tauopathies. Current work focuses on the E3 ligase TRIM11, including the development of therapeutic approaches based on this work, and on identifying new genetic modifiers using the BXD genetic variant lines from The Jackson Labs. A large NIH grant will support this research.
- Mechanisms of tauopathy propagation: This objective explores how tauopathy spreads within the brain and seeks therapeutic strategies to halt it. Key areas of investigation include the movement of tau proteins between neurons, particularly through extracellular vesicles, and the role of cellular mechanisms like proteostasis (protein homeostasis) in tau propagation. New mouse models of tauopathy are being developed to better understand these processes. The first manuscript describing tau KI mice was recently published (Watamura, Foiani et al. 2025 Nature Neuroscience). A manuscript describing tau in extracellular vesicles was also recently published (Fowler, Behr et al. 2024 Nature Neuroscience).
- Selective cellular vulnerability: This objective focuses on why certain types of neurons are more vulnerable to tauopathy. Using advanced techniques such as spatial transcriptomics and proteomics, the research profiles vulnerable neurons and identifies molecular pathways that could be targeted therapeutically. The goal is to understand why excitatory neurons are more prone to tau pathology, or resilient to it, and to develop protective strategies. Spatial transcriptomics protocols (coppaFISH, MERFISH, stereoseq) and single cell proteomics techniques have been optimised and are being used on our new mouse models and human tissue.
- Therapeutic interventions in early disease stages: The final objective involves identifying and testing therapies effective in the earliest stages of tauopathies. We have developed novel human neuron derived cell models as well as slice culture models to help identify novel therapeutics.
The programme is highly collaborative, involving partnerships within UK DRI and globally, leveraging cutting-edge technologies to advance the understanding and treatment of tau-related dementias.
Major projects
Key publications
Vacancies
Lab members
- Mathieu Bourdenx (Senior Postdoctoral Researcher) (funded by Cure Alzheimer's Fund)
- Dr Martha Foiani (Senior Postdoctoral Researcher) (funded by Alzheimer's Association, ARUK)
- Dr Eliona Tsefou (Senior Postdoctoral Researcher) (funded by RCF)
- Dr Nathasia Muwanigwa (Postdoctoral Researcher)
- Dr Tim Birkle (Postdoctoral Researcher)
- Dr James Scott-Solache (Postdoctoral Researcher)
- Raquel Taddei (Postdoctoral Researcher)
- Samantha Henry (Executive Assistant)
- Saisha Patel (PhD Student)
- Izzie Prankerd (PhD Student)
- Paula Cauhy (PhD Student)
- Adnan Avdic-Belltheus (Senior Research Technician)
- Hannah Davies (Technician)
- Luiza-Simona Damoc (Technician)
- Li Wang (Technician)
- Yun Xiang (Student)
Alumni - Dr Stephanie Fowler (now Oxford University)
- Sumi Bez (PhD 2025)
Lab funders
Thank you to all those who support the Duff Lab!