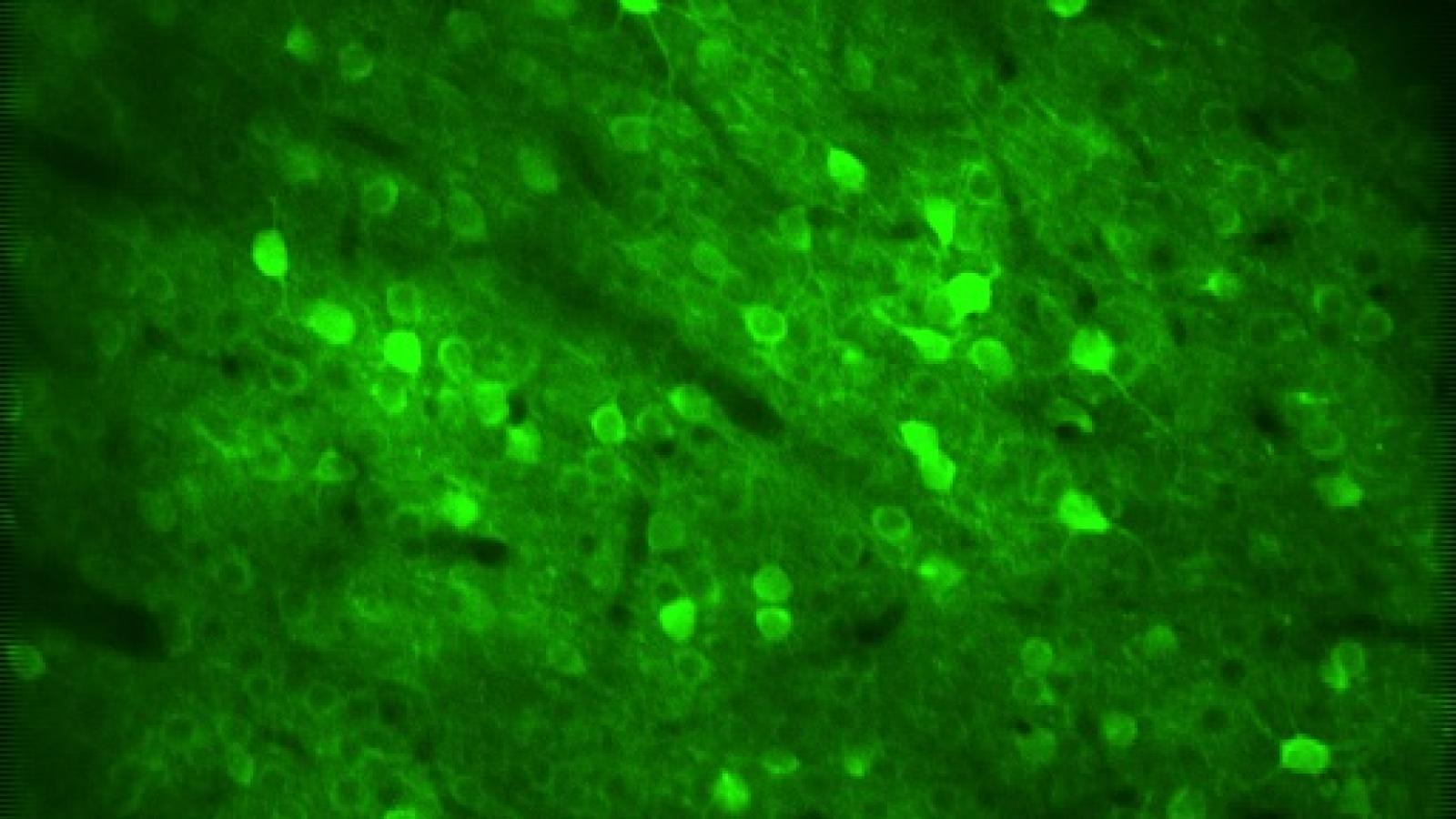
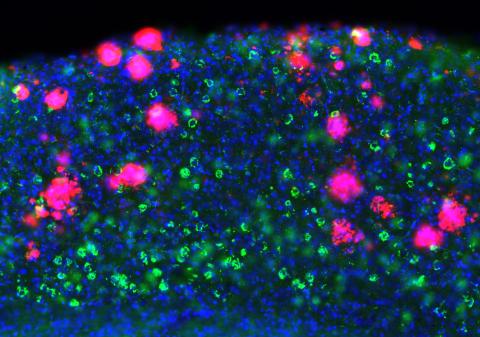
Researchers led by Dr Samuel Harris and Dr Marc Aurel Busche (UK DRI at UCL) have discovered that the amyloid precursor protein (APP) family, known for its role in Alzheimer’s, is essential for healthy communication between brain cells. The study, published in Cell Reports, uncovers a previously unknown role for these proteins in helping sustain normal brain cell activity, and provides new insights into how their disruption could contribute to cognitive impairments associated with Alzheimer’s.
What was the challenge?
APP is best known as the source of amyloid-beta, a protein that misfolds and accumulates in the brains of people with Alzheimer’s. However, the normal function of APP and its related proteins, APLP1 and APLP2, remains poorly understood. The APP family is highly conserved throughout evolution and family members have overlapping roles, pointing to critical biological importance. Scientists have long suspected these proteins play an important role in brain circuits related to memory, but exactly how their absence affects brain cell activity has remained elusive.
What did the team do and what did they find?
Using cutting-edge brain-recording technologies to monitor brain cell activity in real-time, the researchers studied mice that lacked all three APP family members in excitatory neurons - the type of brain cells responsible for activating neuronal circuits involved in memory and cognition. They focused specifically on the neocortex and hippocampus, brain regions critical for learning and memory, to understand how losing these proteins affects neuronal function.
They discovered that neurons lacking the APP family had dramatically decreased activity, or were completely silent, and failed to fire at expected levels. This lack of activity disrupted the ability of neurons to flexibly respond during behaviourally important states. At a molecular level, and informed by computational modelling, these impairments were explained by a significant loss of a type of receptor known as NMDA receptors at synapses, critical for neuron-to-neuron communication, brain function and learning.
Importantly, the researchers found that boosting NMDA receptor function pharmacologically restored activity patterns in the sparsely active neurons and corrected behavioural impairments in the animals.
This discovery highlights a previously underappreciated role of these proteins in healthy brains. Our findings show that APP family proteins have an important physiological role, ensuring that neurons maintain appropriate levels of activity and responsiveness. Without these proteins, the brain struggles to coordinate essential patterns of neuronal activity, with clear impacts on cognitive behaviour.
Group Leader
What is the impact?
This study identifies a previously unknown physiological role for the APP protein family in supporting healthy neuronal communication, especially in brain regions crucial for memory and cognition. By demonstrating a clear mechanistic connection between APP proteins and NMDA receptor function in the living brain, the findings suggest one possible pathway through which altered APP function could contribute to neuronal dysfunction.
These insights highlight the importance of carefully considering the normal roles of APP proteins, particularly since some experimental Alzheimer's therapies aim to reduce APP expression in the brain. While these therapeutic strategies may offer promise in reducing toxic amyloid-beta production, these findings indicate that completely eliminating APP function might disrupt essential neuronal activity.
Thus, future therapeutic approaches targeting APP should endeavour to preserve its beneficial physiological roles while mitigating harmful pathological effects. Striking this careful balance could be key to developing safer, more effective, treatments that protect cognitive function and improve the lives of people living with Alzheimer's.
First author Dr Samuel Harris, Senior Research Fellow, said:
“Uncovering the physiological role of the APP family is essential for understanding how these proteins contribute to brain dysfunction in Alzheimer’s disease and other neurological disorders. Our study adds to the emerging picture of key Alzheimer’s-associated proteins as critical and sensitive modulators of neuronal and circuit function, offering potential insights into the mechanisms that may drive disease progression and heterogeneity, and with implications for future therapeutic strategies.”
Reference: Samuel S. Harris, Rikesh M. Rajani, Jana Zünkler, Robert Ellingford, Mengke Yang, James M. Rowland, Alexander Schmidt, Byung Il Lee, Marten Kehring, Mariam Hellmuth, Francesca Kar Wey Lam, Dominique Fässler, Susanne Erdinger, David P. Wolfer, Carlo Sala Frigerio, Fred Wolf, Bradley T. Hyman, Ulrike C. Müller, Marc Aurel Busche, The amyloid precursor family of proteins in excitatory neurons are essential for regulating cortico-hippocampal circuit dynamics in vivo, Cell Reports, Volume 44, Issue 6, 2025, DOI: 10.1016/j.celrep.2025.115801.
Banner image: Neurons within the cerebral cortex imaged in the living brain in vivo with two-photon microscopy. Credit: James Rowland