New research led by Prof David Hunt and Dr Sarah McGlasson (UK DRI at Edinburgh) sheds light on the mechanisms underlying a rare genetic disorder that affects small blood vessels in the brain and other organs. The study, published in the journal Brain, represents a major step forward in understanding how the condition progresses, and could open up a new treatment approach.
What was the challenge?
Retinal Vasculopathy with Cerebral Leukoencephalopathy and Systemic Manifestations (RVCL-S) is a rare genetic disease of small blood vessels, caused by a mutation in a gene called TREX1. The gene produces an enzyme that trims damaged fragments from the ends of DNA strands. Worldwide, little over 25 families with RVCL-S are known.
There are no disease modifying treatments available for the condition, which affects the small vessels in almost all major organ systems, leading to multiple organ failure and brain disease in the early 40s. Despite uncovering the genetic cause, the underlying mechanisms that lead to the progression of the disease remain unknown.
What did the team do and what did they find?
By analysing genetic sequencing data from over 400,000 UK Biobank participants, the researchers found that for the TREX1 mutation to cause RVCL-S, the enzyme must be active.
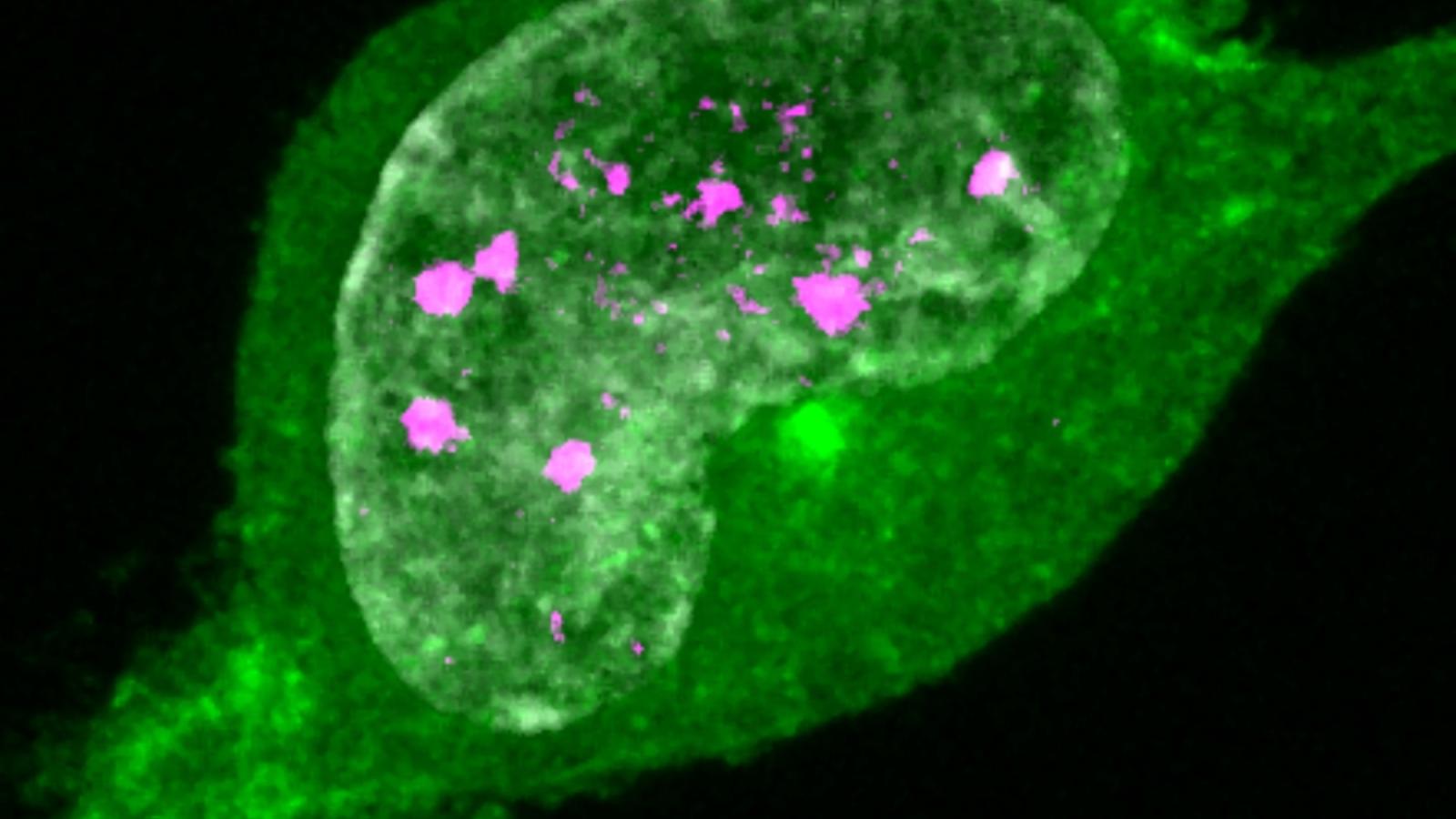
The team discovered that RVCL-S-causing mutations disrupt how TREX1 interacts with other proteins. This leads the enzyme to end up being directed to the wrong place within cells, frequently ending up in the nucleus, which contains the cell’s genetic material. The researchers showed that, in endothelial cells – the cells that line blood vessels – with a patient mutation, this causes DNA damage, as the enzyme cleaves strands of DNA. By blocking the activity of TREX1 in the cells, the team were able to prevent DNA damage from occurring.
Using a multidisciplinary approach, our study has revealed that RVCL is caused by DNA damage within the small bloods vessels. This is important because it opens up new drug targets for this rare but serious brain disorder. It also highlights a new mechanism of small vessel disease which could help us better understand vascular dementias.
Dr Sarah McGlassonUK DRI postdoctoral researcher and Clayco RVCL Fellow
What is the impact?
The research identifies an important therapeutic target in RVCL-S. Blocking the activity of the untethered TREX1’s activity can prevent DNA damage, opening up a new potential treatment approach for people affected by RVCL-S. The researchers also hope that identifying how endothelial DNA damage can cause small vessel disease could be more broadly relevant to vascular dementias.
This study was supported by the Clayco Foundation.
Reference: Sarah McGlasson, Katy Reid, Anna Klingseisen, Bastien Rioux, Samuel Chauvin, Cathrine A Miner, Joe Holley, Deborah Forbes, Bethany Geary, Jeffrey Kimber, Katrina Wood, Candice Roufosse, Colin Smith, David Kavanagh, Jonathan Miner, David P J Hunt, Misdirected yet intact TREX1 exonuclease activity causes human cerebral and systemic small vessel disease, Brain, 2025;, awaf085, https://doi.org/10.1093/brain/awaf085