Scientists led by Prof Tara Spires-Jones and Dr Claire Durrant (UK DRI at Edinburgh) have discovered a new drug target for Progressive Supranuclear Palsy (PSP). The study, published in Nature Neuroscience, could lead to future therapeutics for the condition.
What was the challenge?
PSP is a rare neurodegenerative disease that causes problems with movement, thinking, and behaviour. People typically develop PSP in their mid to late 60s, and symptoms worsen rapidly, causing severe disability within 5 years of symptom onset. There are currently no treatments that can slow or stop PSP progression.
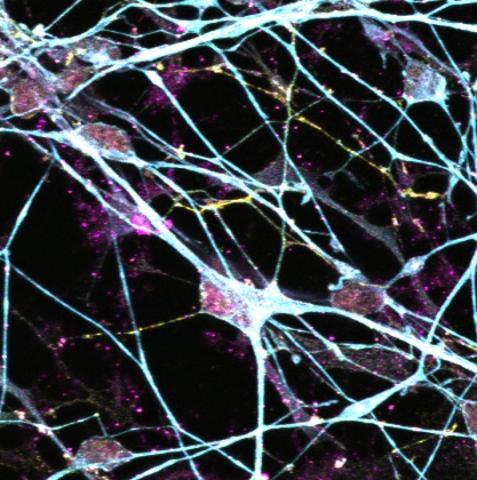
Toxic forms of tau protein clump inside neurons in people with PSP and spread through the brain. Scientists have shown that synapses, the connections between neurons, die in PSP in the areas of the brain affected by toxic tau. However, it is not known whether tau directly affected synapses or how the clumps of tau spread through the brain.
What did the team do and what did they find?
In this study, neuroscientist and veterinarian Dr Robert McGeachan observed tau inside synaptic connections in donated post-mortem brain tissue samples from people who died with PSP. The presence of tau in synapses was associated with synapse death, meaning the tau was likely toxic and killing these connections.
Digging deeper, the team found evidence that small clumps of tau may be spreading through the brain by jumping through synaptic connections, from pre-synapse, to post-synapse. To examine whether this jumping of tau through synapses could be happening in living human brain tissue, the team used a new method pioneered by Dr Claire Durrant in collaboration with neurosurgeon Prof Paul Brennan.
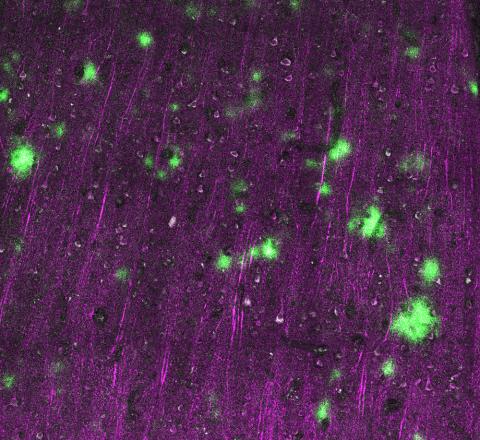
The researchers challenged living human brain slices with tau protein taken from post-mortem brain tissue of people who died with PSP. Post-synapses in the living brain slices took up the toxic PSP tau, supporting the idea that tau may jump to neurons by sneaking into post-synapses. This tau challenge triggered synapses to be eaten by astrocytes, support cells in the brain, further confirming that synaptic tau is toxic.
This pattern of tau in synapses strongly suggests that tau may be jumping from one side of the synapse to the other. Since neurons make synapses with other neurons in distant brain regions, this jumping could explain why tau pathology spreads through the brain between regions that have synaptic connections. Targeting tau in synapses may help to slow disease progression in PSP.
Group Leader & Research Division Lead
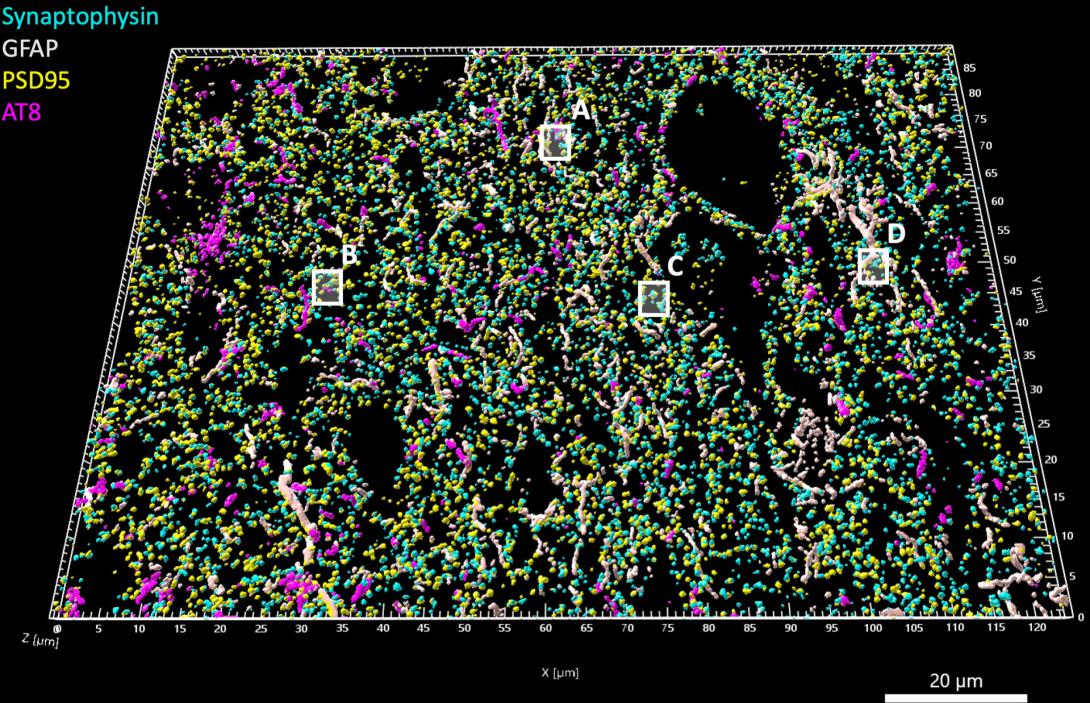
3D reconstruction of high-resolution array tomography images of the frontal cortex from a person who died with Progressive Supranuclear Palsy. Magenta = Tau, Cyan = Presynaptic terminals, Yellow = Postsynaptic terminals, and Grey = Astrocytes. Credit: Robert McGeachan
The team also found a protein that may play a role in tau toxicity and spreading. Clusterin, a protein that has been implicated in Alzheimer’s disease, was found in the same synapses as toxic tau, in PSP brain samples. Super-resolution imaging showed that clusterin and tau are close enough to be interacting inside post-synapses, giving a tantalizing hint that this protein could be involved in degeneration or tau spreading in PSP.
What is the impact?
While these new data are a long way from a drug to treat people living with PSP, they do expand our understanding of the disease and provide new targets to develop towards effective treatments. Targeting tau to stop synapses from dying and stop pathology from spreading through the brain is a promising approach for future treatments.
Dr Claire Durrant said:
"By using both post-mortem PSP brain samples, alongside live human brain slice cultures treated with PSP-derived tau, this study gives us insight into how pathological tau may spread through, and damage, the brain in PSP. As dementia research moves closer towards developing treatments for these devastating diseases, the use of human tissue will be increasingly important to ensure preclinical findings have the best chance of working in patients."
Dr Robert McGeachan said:
“Progressive Supranuclear Palsy is a devastating disease not only for those living with it, but also for their families, friends, and carers. Due to its rapid progression, it can sadly take away a person’s independence in just a few short years. This research provides crucial new insights into how the disease spreads in the brain, bringing us one step closer to effective treatments that could slow or prevent progression.”
Reference: McGeachan, R.I., Keavey, L., Simzer, E.M. et al. Evidence for trans-synaptic propagation of oligomeric tau in human progressive supranuclear palsy. Nat Neurosci (2025). https://doi.org/10.1038/s41593-025-01992-5