Key details
Employing antivirals to clear protein aggregates
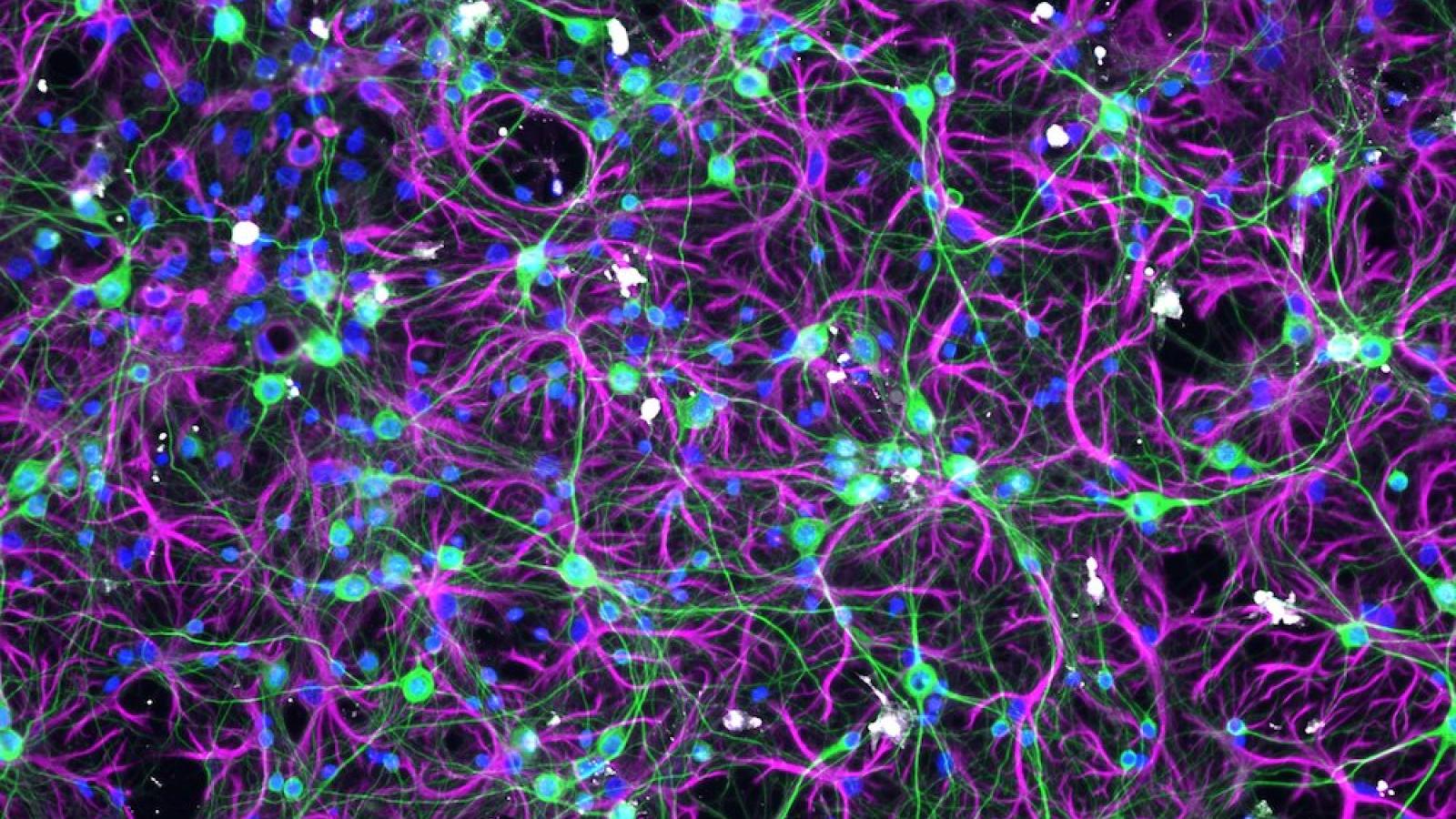
During neurodegenerative diseases, specific proteins such as tau accumulate into clumps or 'aggregates', causing the cells of our brain to malfunction and die. Clearing such protein aggregates is difficult, but could offer methods to reduce the burden of disease.
The McEwan Lab is examining cells’ intrinsic ability to fight infectious agents like viruses, and attempting to use these methods to destroy protein aggregates. By redirecting an antiviral protein called TRIM21, they have shown for the first time that existing tau aggregates can be removed from inside cells. The team are investigating such mechanisms for a better understanding of disease and as potential routes to new therapies.
Latest news

Prof Will McEwan
Prof Will McEwan is a Group Leader at the UK DRI at Cambridge. Find out more about his career and expertise on his profile page.

Research summary
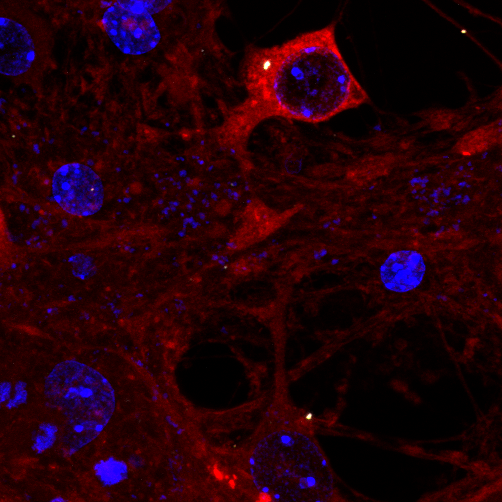
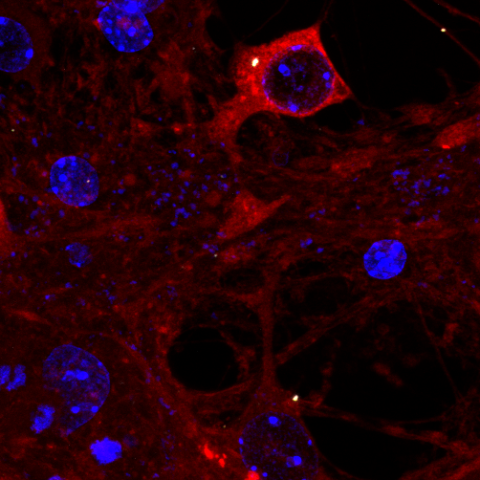
Tau assembly being attacked by TRIM21 inside a neuron. Credit: Annabel Smith
Protein misfolding and its interception by intrinsic immune responses
Cytosolic protein aggregates are almost universally present in neurodegenerative diseases but are considered challenging substrates for conventional degradation pathways. Cytosolic intrinsic immune pathways have evolved to degrade large proteinaceous particles such as viruses and therefore offer an opportunity for repurposing to destroy aggregated proteins. Prof Will McEwan's team discovered that the cytosolic antibody Fc receptor, TRIM21, can be used to degrade antibody-coated tau assemblies, opening new possibilities for immunotherapy against tau. Following this, we are exploring new mechainsms by which large protein aggregates can be marked for degradation. The lab also undertake basic biology research to better understand the mechanisms of tau aggregation and its broader interaction with the innate immune system, particularly the type I interferon response. They develop and use a range of cell-based, ex vivo and in vivo models in which to investigate protein aggregation.
The objectives of the McEwan lab are:
- Developing potent and selective mechanisms of protein aggregate degradation
- Devising new tau immunotherapies that are more effective and safer than previous generations
- Understanding how tau propagates in the human brain
- Testing the role of innate immunity in promoting tau pathology
Vacancies
Key publications
Lab members
- Dr Aamir Mukadam (Postdoctoral Researcher)
- Sumi Bez (Postdoctoral Researcher)
- Sophie Keeling (Research Assistant)
- Annabel Smith (Research Assistant)
- Anna Brown (Research Assistant)
- Matthew Reid (Research Assistant)
- Ella Lacey (PhD Student)
Collaborators





Lab funders
Thank you to all those who support the McEwan Lab!