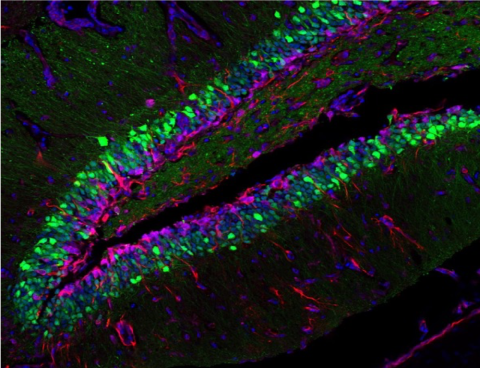
The aim of the UK DRI at King’s is to understand the very earliest molecular and pathophysiological events that initiate neurodegeneration and develop novel therapeutic strategies. Focusing on amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), researchers at the Centre aim to study the role of genes and molecular pathways involved in neurodegeneration.
Groundbreaking work at the Centre has revealed a key role for RNA-binding proteins in the onset and progression of ALS and FTD, which commonly mislocalise into cytoplasm and aggregate in affected cells. It remains to be determined how these defects deregulate cellular RNAs, and how such deregulation is linked to altered energy metabolism and other pathways implicated in these diseases through genetic risk variants or environmental responses. The teams aim to answer key questions such as:
- Which cell types are vulnerable to the genetic and environmental variables that drive ALS/FTD?
- What role does disrupted RNA regulation and energy metabolism play in the shared pathomechanisms?
- Can we understand the causes of the aggregation and toxicity of key RNA-binding proteins via their altered RNA binding, liquid-liquid phase separation and nucleo-cytoplasmic transport?
- Can we therapeutically target the shared pathomechanisms across genetic and sporadic forms of disease?
- Can we enhance endogenous neuroprotective pathways for novel therapeutic approaches?
The UK DRI at King’s has a clear translational trajectory, as researchers are designing, manufacturing, patenting, and testing RNA- and gene therapy-based potential therapeutics in preclinical models. In 2019, a spinout, AviadoBio, was launched from the Centre, co-led by Prof Chris Shaw, to develop innovative gene therapies to treat ALS and FTD.
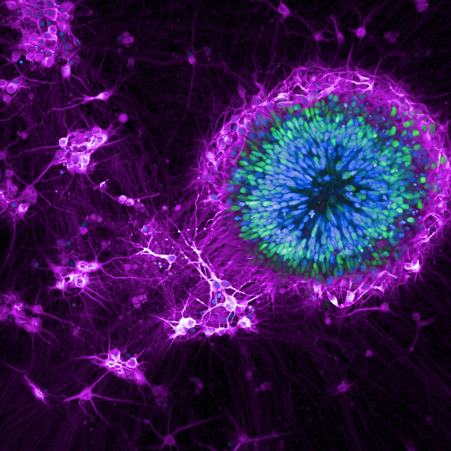
iPSC cells used in MND/FTD research. Credit: Mizielinska Lab